CoviDx™ SARS-CoV-2 Rapid Antigen Test
$ 125.00
Available in a box of 25 tests at $125.00 ($5.00 / test)
Our CoviDx™ SARS-CoV-2 Rapid Antigen Test is a rapid screening test that detects the presence of the COVID-19 antigen.
CoviDx™ SARS-CoV-2 Rapid Antigen Test is a lateral flow assay intended for the qualitative detection of the nucleocapsid protein antigen from SARS-CoV-2 in nasal swabs and nasopharyngeal swabs from patients suspected of a Coronavirus-19 (COVID-19) infection by their healthcare provider within the first 5 days of symptoms.
Out of stock
Add to quote requestThis test is authorized for use at the point-of-care, i.e., in patient care setting. It can also be administered in the workplace to employees by health professionals such as doctors, nurses and other qualified professionals or administrative staff who have received prior training. Non-medical staff can only perform nasal tests.
One box of the CoviDx™ SARS-CoV-2 Rapid Antigen Test will allow you to test 25 people.
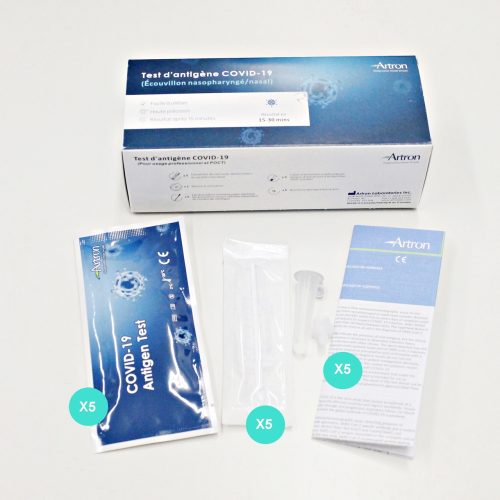
What’s in the box
- 25 individually sealed test devices
- 25 individually sealed swabs
- 25 pre-filled extraction vials
- 25 nozzles
- Tube stand
- Package Insert
Store between 2 and 30°C / 36 and 86°F
Not included, but needed before performing a test
- Personal protective equipment (gloves, mask, face shield, isolation gown, disinfectant, etc.)
- Timer
- Biohazard trash can (for medical waste)
- Paper towels
Results obtained in just 20 minutes.
- The procedure is quick and easy to perform and only takes a few minutes.
- When completed, you just have to wait 20 minutes to get the result (positive or negative).
The results obtained therefore allow the identification of the viral nucleoprotein antigen of SARS-CoV-2 which is generally detectable in nasopharyngeal and nasal secretions during the acute phase of the infection. Positive results indicate the presence of viral antigens, but clinical correlation with the patient’s history and other diagnostic information is needed to determine the status of the infection.
If the results are positive, the person may have a bacterial infection or co-infection with other viruses. The agent detected may not be the definitive cause of the disease. Laboratories are required to report all positive results to the appropriate public health authorities. Negative results should be treated as presumptions and do not rule out SARS-CoV-2 infection and should not be used as the sole basis for treatment or patient management decisions, including decisions infection control. Negative results should be considered in the context of a patient’s recent exposures, history and presence of clinical signs and symptoms compatible with COVID-19, and confirmed by molecular testing, if necessary, for the management of the patient.
CoviDx™ SARS-CoV-2 Rapid Antigen Test Disclaimer
By purchasing these tests, you agree to follow the following conditions:
- Health Canada regulations and limits of use
In accordance with Health Canada regulations and under article 5 of the provisional order concerning the sale and import of medical devices to be used for COVID-19, taken by the Minister of Health on March 18, 2020, the COVID-19 CoviDx™ SARS-CoV-2 Rapid Antigen Test is now authorized for sale or import in Canada. This agreement is intended to ensure that you receive, understand and comply with the terms of these authorizations and the corresponding product inserts. The authorization reference number for the Covid-19 CoviDx™ SARS-CoV-2 Rapid Antigen Test is 330636.
Without limiting ourselves to the following, we wish to clarify for each test device certain notable points of these authorizations and the product instructions:
Authorized users
- These tests are intended to be used at the point of service for business purposes only.
- The COVID-19 CoviDx™ SARS-CoV-2 Rapid Antigen Test is authorized for nasal and nasopharyngeal secretions collected at the point of care.
Additional notifications
- These tests were authorized by Health Canada under provisional orders.
- In vitro diagnostic tests intended for professional use only.
- The CoviDx™ SARS-CoV-2 Rapid Antigen Test should only be used for the qualitative detection of SARS-CoV-2 antigens.
- These authorizations are only valid as long as the provisional order concerning the sale and the import of medical devices to be used for COVID-19 is in effect.
- Reporting requirements may differ depending on guidelines issued by local health authorities.
THE CRITERIA SET OUT IN THESE AUTHORIZATIONS AND PRODUCT NOTICES ARE APPLICABLE TO THE SALE, RESALE, AND USE OF THE CoviDx™ SARS-CoV-2 Rapid Antigen Test AND ARE NOT LIMITED TO THE MENTION ABOVE ONLY.
On reading the above, and as a registered authorized purchaser for your business, you accept this Agreement, acknowledging receipt and confirming compliance with the terms described above. As an authorized person representing the company and the affiliated entities of this company, you agree to indemnify and hold Alco Prevention Canada harmless. for all fines, damages, losses, costs and expenses incurred as a result of any breach of this Agreement caused by you or by a third party who received this product through your business.
Ask our staff for more information.
By purchasing this product, you agree to follow the manufacturer’s intended use and the sale conditions in this document and the sale conditions of this website.
We recommend that you contact the distributor of your choice to ensure the availability of the desired product.
Reviews
Write a customer review
This test is authorized for use at the point-of-care, i.e., in patient care setting. It can also be administered in the workplace to employees by health professionals such as doctors, nurses and other qualified professionals or administrative staff who have received prior training. Non-medical staff can only perform nasal tests.
One box of the CoviDx™ SARS-CoV-2 Rapid Antigen Test will allow you to test 25 people.
What’s in the box
- 25 individually sealed test devices
- 25 individually sealed swabs
- 25 pre-filled extraction vials
- 25 nozzles
- Tube stand
- Package Insert
Store between 2 and 30°C / 36 and 86°F
Not included, but needed before performing a test
- Personal protective equipment (gloves, mask, face shield, isolation gown, disinfectant, etc.)
- Timer
- Biohazard trash can (for medical waste)
- Paper towels
Results obtained in just 20 minutes.
- The procedure is quick and easy to perform and only takes a few minutes.
- When completed, you just have to wait 20 minutes to get the result (positive or negative).
The results obtained therefore allow the identification of the viral nucleoprotein antigen of SARS-CoV-2 which is generally detectable in nasopharyngeal and nasal secretions during the acute phase of the infection. Positive results indicate the presence of viral antigens, but clinical correlation with the patient’s history and other diagnostic information is needed to determine the status of the infection.
If the results are positive, the person may have a bacterial infection or co-infection with other viruses. The agent detected may not be the definitive cause of the disease. Laboratories are required to report all positive results to the appropriate public health authorities. Negative results should be treated as presumptions and do not rule out SARS-CoV-2 infection and should not be used as the sole basis for treatment or patient management decisions, including decisions infection control. Negative results should be considered in the context of a patient’s recent exposures, history and presence of clinical signs and symptoms compatible with COVID-19, and confirmed by molecular testing, if necessary, for the management of the patient.
CoviDx™ SARS-CoV-2 Rapid Antigen Test Disclaimer
By purchasing these tests, you agree to follow the following conditions:
- Health Canada regulations and limits of use
In accordance with Health Canada regulations and under article 5 of the provisional order concerning the sale and import of medical devices to be used for COVID-19, taken by the Minister of Health on March 18, 2020, the COVID-19 CoviDx™ SARS-CoV-2 Rapid Antigen Test is now authorized for sale or import in Canada. This agreement is intended to ensure that you receive, understand and comply with the terms of these authorizations and the corresponding product inserts. The authorization reference number for the Covid-19 CoviDx™ SARS-CoV-2 Rapid Antigen Test is 330636.
Without limiting ourselves to the following, we wish to clarify for each test device certain notable points of these authorizations and the product instructions:
Authorized users
- These tests are intended to be used at the point of service for business purposes only.
- The COVID-19 CoviDx™ SARS-CoV-2 Rapid Antigen Test is authorized for nasal and nasopharyngeal secretions collected at the point of care.
Additional notifications
- These tests were authorized by Health Canada under provisional orders.
- In vitro diagnostic tests intended for professional use only.
- The CoviDx™ SARS-CoV-2 Rapid Antigen Test should only be used for the qualitative detection of SARS-CoV-2 antigens.
- These authorizations are only valid as long as the provisional order concerning the sale and the import of medical devices to be used for COVID-19 is in effect.
- Reporting requirements may differ depending on guidelines issued by local health authorities.
THE CRITERIA SET OUT IN THESE AUTHORIZATIONS AND PRODUCT NOTICES ARE APPLICABLE TO THE SALE, RESALE, AND USE OF THE CoviDx™ SARS-CoV-2 Rapid Antigen Test AND ARE NOT LIMITED TO THE MENTION ABOVE ONLY.
On reading the above, and as a registered authorized purchaser for your business, you accept this Agreement, acknowledging receipt and confirming compliance with the terms described above. As an authorized person representing the company and the affiliated entities of this company, you agree to indemnify and hold Alco Prevention Canada harmless. for all fines, damages, losses, costs and expenses incurred as a result of any breach of this Agreement caused by you or by a third party who received this product through your business.
Ask our staff for more information.
By purchasing this product, you agree to follow the manufacturer’s intended use and the sale conditions in this document and the sale conditions of this website.
We recommend that you contact the distributor of your choice to ensure the availability of the desired product.
Reviews
Write a customer review